Febrile seizures (FSs) are the most common type of childhood seizures, occurring in 3% to 5% of children between 6 months and 6 years of age, with peak incidence in the second year of life. They are familial in some cases and sporadic in others, suggesting both genetic and environmental factors play a role.
A sudden rise in temperature is often described, and FSs are most commonly associated with a febrile viral illness. They are frightening to parents and often lead to medical consultation. In addition, ∼30% of children with a first FS will have a second episode, with risk factors for recurrence being younger age at first FS and family history of FS. Epidemiological studies reveal that most children with a history of FS have normal behavior, intelligence and academic achievement, and do not later develop epilepsy.
Whole-cell pertussis and measles-containing vaccines as well as some influenza vaccines in combination with pneumococcal vaccines are associated with an increased rate of FSs within a defined period of time after vaccination when fever peaks. FS associated with a vaccination can decrease parent and provider confidence in vaccine safety and impact future vaccination of the child and other family members. When 1 seasonal influenza brand in Australia was withdrawn in 2010 because of increased risk of FS, it led to an overall reduction in influenza vaccine confidence and coverage despite no further FS signal being detected in subsequent years. While that particular influenza vaccine was associated with significant sequelae, it is unclear whether other vaccine-proximate febrile seizures (VP-FSs), occurring within a time frame when the fever may have been caused by vaccination, are any different to FSs due to another cause.
Although data to define the attributable risk of VP-FS are becoming increasingly available, only 2 previous studies, within the same cohort of US children aged 6 months to 3 years, directly compared VP-FS to non–vaccine-proximate febrile seizure (NVP-FS). In the first study, children with a first VP-FS were more likely to be girls, younger, have a lower birth weight, a lower Apgar score at 1 minute, and a higher chance of FS recurrence compared with children with NVP-FS. The second study revealed no difference in risk of hospitalization for first FS. However, the authors of these retrospective studies did not examine other markers of seizure severity such as duration, recurrence within the same admission, or use of antiepileptics. The effect of a laboratory-confirmed coexisting infection on VP-FS has also never been examined. We conducted a prospective cohort study of children aged ≤6 years to examine differences in and contributors to first FS severity and FS recurrence in the 6 months after the initial FS presentation in VP-FS and NVP-FS cases.
Methods
Case Ascertainment and Study Population
Active prospective FS surveillance was conducted from May 1, 2013, to June 30, 2014, through the Pediatric Active Enhanced Disease Surveillance (PAEDS) Network at 5 Australian tertiary hospitals: the Children’s Hospital at Westmead Sydney, Royal Children’s Hospital Melbourne, Princess Margaret Hospital for Children Perth, Women’s and Children’s Hospital Adelaide, and Lady Cilento Children’s Hospital Brisbane, as previously described in another study using the same study cohort.
Specialized surveillance nurses systemically identified potential FS cases by screening emergency department and inpatient databases and reviewing all records with International Classification of Diseases, 10th Revision, Australian Modification diagnosis code for FS (R56.0).
Children aged ≤6 years were included in the study if they presented with their first seizure, where the seizure fulfilled the Brighton Collaboration case definition and was associated with a fever, defined as a temperature of >38°C, reported by their caregiver or documented by paramedics or health care worker on presentation to the hospital. Per the International League Against Epilepsy definition of FS, children were excluded if they had a previous seizure and/or existing neurologic condition reported by their caregiver or if they were found to have a central nervous system infection by cerebrospinal fluid (CSF) analysis.
Clinical details were collected through caregiver interviews and included age at time of FS, aboriginal and Torres Strait Islander status, country of birth (Australia or other), birth weight, gestational age at birth, history of meningitis or encephalitis or other chronic medical conditions, family history of FSs or epilepsy, and clinical symptoms on seizure presentation. Investigations when performed included blood, urine, CSF culture, nasopharyngeal aspirate (NPA), EEG, and imaging (computed tomography [CT] or MRI), with these results being obtained through medical record review. Subsequent FS presentations of the same child within the study period were also recorded. Receipt of immunizations were verified for all children by using data from the Australian Immunization Register.
Participants recruited between May 1 and December 31, 2013, were contacted via phone to assess FS recurrence 6 months after the initial FS presentation. Because of study resource constraints, follow-up of cases recruited between January 1 and June 30, 2014, was not performed.
Case Definitions and Outcome Measures
Було проведено статистичний аналіз 1022 випадків госпіталізації дітей, від 6 місяців до 6 років від роду, з приводу фебрильних судом, що сталися вперше. При цьому, випадок ФС враховувався, як пов’язаний із вакциOn the basis of previous studies on timing of fever onset after specific vaccines, VP-FS was defined as an FS that occurred from day 0 to 2 after receipt of an inactivated vaccine, day 5 to 14 after a live-attenuated vaccine, or day 0 to 14 after a combination of inactivated and live-attenuated vaccines. An FS outside of this period was considered an NVP-FS.нацією у разі дотримання трьох умов:
The primary outcome measures were seizure severity defined as seizure duration >15 minutes, further seizures in the subsequent 24 hours, and antiepileptic drug (AED) use; secondary outcome measures were length of stay (LOS) in hospital >1 day, transfer from a peripheral hospital, ICU admission, death, and readmission for FS recurrence within 48 hours of initial FS.
Cases were defined as having a coexisting infection if ≥1 laboratory investigations (blood, urine or CSF culture, CSF polymerase chain reaction, or NPA polymerase chain reaction) detected viral or bacterial pathogens. Investigations performed on readmission within 48 hours of initial presentation were considered as the same illness and were combined with any initial investigations in the analysis. Investigations were performed at the clinicians’ discretion.
Statistical Analysis
Demographic data on and reported symptoms from patients with VP-FS and NVP-FS were compared by using a χ2 or Fisher’s exact test for categorical values, as appropriate, and the Mann-Whitney U test for nonparametric continuous values. Logistic regression was performed for each clinical outcome measure, with the exposure of interest categorized as either VP-FS or NVP-FS and adjusted for age categories (<12, 12–24, 24–36, ≥36 months) and sex. VP-FS cases with coinfection were compared with cases with no coinfection or not tested by using Fisher’s exact test. Statistical analyses were performed with SAS (SAS Institute, Inc, Cary, NC) version 9.3.
Results
Patient Characteristics
There were 1735 potential FS episodes in 1504 children aged 0 to 6 years identified through screening between May 1, 2013, and June 30, 2014, across the 5 PAEDS sites. Twenty-one patients with a previous afebrile seizure, 45 with an existing neurologic condition, and 7 confirmed meningitis cases were excluded from the study. Of the 1662 FS cases remaining, 640 were excluded because they were not the first FS episode, leaving 1022 first FS cases of which 67 (6%) were VP-FSs and 955 (94%) were NVP-FSs. A subset of 638 cases recruited between May 1 and December 31, 2013, were contacted for follow-up at 6 months, and 398 responded (62% overall response rate, 62% [373 of 598] for NVP-FS; 63% [25 of 40] for VP-FS).
Children with their first VP-FS were younger than children with their first NVP-FS (13 vs 20 months; P < .001)(Table 1). There was no difference in family history of FS or epilepsy between VP-FS and NVP-FS groups. There were no differences in birth weight, gestational age at birth, country of birth, Aboriginal and/or Torres Strait Islander background, or past medical history of meningitis or encephalitis or other chronic medical conditions between the 2 groups.
VP-FS
Of the 67 VP-FS cases, 56 (84%) were after vaccination with measles-containing vaccines (of which 40 were measles-mumps-rubella [MMR] with Haemophilus influenzae type b and meningococcal C conjugate [Hib-MenC] vaccine, 12 measles-mumps-rubella-varicella [MMRV], 3 MMR with diphtheria-tetanus-acellular pertussis and inactivated polio combination vaccine [DTaP-IPV], and 1 MMR only). The remaining 11 VP-FSs occurred after diphtheria-tetanus-acellular pertussis, H influenzae type b, hepatitis B, and inactivated polio combination vaccine (DTaP-Hib-HepB-IPV) with 13-valent pneumococcal conjugate vaccine (PCV13) and rotavirus (n = 7), varicella (n = 2), DTaP-Hib-HepB-IPV (n = 1), and influenza (n = 1) vaccines. The peak incidence of FS was 9 days postvaccination, of which 13 were after vaccination with MMR and 1 after MMRV (Fig 1).
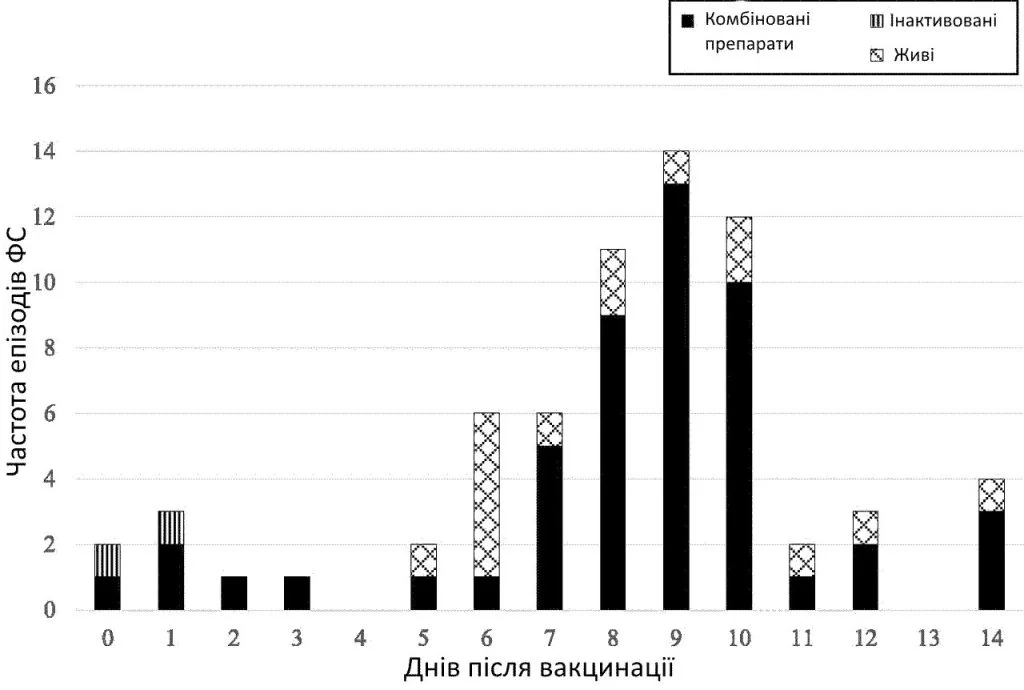
Timing of first FS after vaccination by type of vaccination received.
Seizure Severity and Outcome
Univariate and multivariate analyses revealed no increased risk of a severe seizure associated with a VP-FS compared to an NVP-FS (Table 2). Most VP-FSs and NVP-FSs were short (≤15 minutes) with a LOS of 1 day or less, and no differences in FS recurrence within the first 24 hours of the initial FS were observed. There was an increased risk of AED use for seizure termination for VP-FSs compared to NVP-FSs (adjusted odds ratio [aOR] 2.24; 95% confidence interval [CI] 1.07–4.67; P = .03) but no difference at discharge. An AED was used for seizure termination in all 10 cases of prolonged VP-FS compared to only 47 cases (59%) of prolonged NVP-FS. Children with VP-FS were more likely to be transferred from a peripheral hospital than children with NVP-FS (aOR 2.36; 95% CI 1.09–5.11; P = .03). Compared to the VP-FS group overall, the 9 VP-FS cases requiring transfer had a higher proportion of patients with prolonged seizures (44% vs 15%), repeat seizures within 24 hours of initial (33% vs 9%), and AED use for initial management (56% vs 15%).
There was no FS recurrence within 48 hours after all first VP-FS. There was also no increased risk of FS recurrence 6 months after the initial VP-FS compared to NVP-FS (aOR 1.17; 95% CI 0.46–2.94; P = .75) in the subset of 398 patients followed-up at 6 months (Table 2).
Clinical Symptoms and Investigation of Outcomes
Respiratory symptoms were the most commonly reported symptom, with similar proportions in each group (62.7% VP-FS vs 62.8% NVP-FS). There was also a similar proportion of patients in each group who had a rash (9.0% vs 6.6%) or irritability and/or lethargy (11.1% vs 8.6%). Vomiting and diarrhea were less frequently reported in VP-FS than in NVP-FS cases (vomiting: 3.7% vs 22.0%; diarrhea: 7.5% vs 11.6%).
Laboratory investigations were performed in a subset of patients at the treating clinicians’ discretion (24% of VP-FS versus 35% of NVP-FS had 1 or more laboratory tests; P = .3). A larger proportion of children with prolonged seizures (56% [61 of 108] vs 33% [285 of 880]; P< .001) or repeat seizures within 24 hours of the initial FS (74% [71 of 96] vs 31% [274 of 892]; P< .001) had investigations performed. FS cases with respiratory symptoms were less likely to have investigations, although the difference was not statistically significant for VP-FS cases. There was no difference in other reported symptoms comparing VP-FS cases that had laboratory investigation to VP-FS cases that did not.
Laboratory-confirmed infection was found in similar proportions in those tested in both groups (30% VP-FS versus 28% NVP-FS; P = .82) (Table 3). Eight out of 27 VP-FS cases tested had a laboratory-confirmed infection (5 respiratory illnesses, 2 Escherichia coli urinary tract infections, and 1 enterovirus gastroenteritis). Four were after the first dose of MMR and 4 after the combination of DTaP-Hib-HepB-IPV, PCV13, and rotavirus vaccine.
Patients with VP-FS with a coinfection were younger compared with patients with VP-FS without coinfection and those not tested (9.8, 13.8, 13.2 months, respectively; P = .02), and a larger proportion required an LOS >1 day (75%, 26%, 2.5%, respectively; P < .001).
Six (9%) VP-FS and 41 (5%) NVP-FS cases had either EEG and/or CT or MRI on the brain. All VP-FS cases that had an EEG or imaging were either prolonged or recurrent FS cases.
Discussion
We present a comprehensive comparison of seizure severity between young children with VP-FS and NVP-FS that should be valuable for counseling parents of children, who, in Australia, will have received 13 vaccinations by the time they reach 2 years of age as part of the National Immunization Program. Our study reveals that VP-FSs are no different in seizure severity to NVP-FSs, with the majority being brief (1 day), and not requiring AED use at discharge.
Our study supports the findings of Tartof et al’s retrospective cohort study, which also revealed no difference in LOS between VP-FS and NVP-FS. Using detailed individual clinical note review, we have better defined the severity of VP-FS with our study. We expand on the Tartof et al study to demonstrate no difference in other clinical severity measures, including rate of ICU admission, seizure duration, recurrence within the initial 24 hours, and requirement of AED use at discharge, which has not been studied before. With our study, we are the first to report no increased risk of prolonged or recurrent FS after VP-FS compared to NVP-FS even after adjusting for age and sex. We found the higher proportion of VP-FS cases transferred from peripheral hospitals compared to NVP-FS cases was associated with other markers of seizure severity, with a higher proportion of children with prolonged seizures or recurrence within the initial 24 hours being transferred. We also found a higher proportion of AED use for seizure termination in VP-FS cases. An AED was used for seizure termination in all prolonged VP-FSs, in accordance to international acute seizure management guidelines, compared to only 59% of prolonged NVP-FSs. It is unclear whether there was a difference in semiology or duration of the prolonged seizures in either group, which may account for the difference in AED use for seizure cessation. Reassuringly, we found no difference in risk of prolonged seizures or the requirement of AED use on discharge between VP-FS and NVP-FS.
The majority of VP-FSs in our study were after measles-containing vaccines, in keeping with a known twofold risk in FS after measles vaccination. They were mostly after the first dose of MMR, and because the first dose of MMR is given at 12 months of age in Australia, this has caused a left shift to a younger mean age of first FS in VP-FS compared to NVP-FS (mean age 13 vs 20 months; P < .0001). A similar age difference between groups was seen in the Tartof et al study.
To our knowledge, this is the first study used to examine the presence of clinical symptoms and the effect of coexisting infections on VP-FS. We identified a large proportion (63%) of VP-FS cases with respiratory symptoms and some with vomiting, diarrhea, or abdominal pain, suggesting some may have an infective contributory cause of the FS in addition to a vaccine. Authors of previous studies examining the risk of vaccines and seizures have not reported on the presence of concomitant infection. It is not possible to determine whether an infection or vaccine is the dominant cause of the FS; however, it is reassuring that the presence of these infective symptoms did not impact seizure severity of VP-FS compared to NVP-FS. Of the 12% of VP-FS cases with laboratory-confirmed coinfection, the only clinical difference was a longer LOS compared with those with no laboratory-confirmed coinfection because of the need for treatment of the underlying infection. Because less than half of VP-FS cases were investigated for infection, it is possible that the proportion of VP-FS with a coinfection is underestimated, and the proportion of FS that is solely attributable to vaccination is lower than previously reported where only the temporal relation with vaccination was considered.
Although risk factors for FS such as family history, prematurity, and fetal growth retardation have been well documented, we did not find any differences in sex, birth weight, or gestational age between VP-FS and NVP-FS, which contrasts with Tartof et al study findings. Study population differences may have contributed to the difference in findings because Tartof et al only included first FS occurring at <3 years of age and more broadly defined VP-FS as an FS 0 to 15 days after any vaccine. The absence of differences in our study is reassuring given our more biologically plausible VP-FS definition and wider capture of all FSs up to 6 years of age that is more in line with FS incidence.
In those followed up to 6 months, the recurrence rate in both VP-FS (23.6%) and NVP-FS (28%) was slightly lower than the 30% recurrence rate reported in previous FS studies. The short follow-up period and small sample size may account for this difference. Although earlier onset of FS is a risk factor for recurrence, there was no increased risk in the younger VP-FS group compared with the NVP-FS group. This was also reported by Tartof et al, whose study had a longer mean follow-up duration of 2.2 years.
The strength of this study lies in the prospective case ascertainment through an established robust active surveillance network in which comprehensive clinical data were collected for analysis. Our strict case definition for VP-FS accounted for differences in fever risk window of specific vaccines allowing for more accurate delineation between VP-FS and NVP-FS. Our ability to collect clinical symptoms and investigation data allowed us to examine the impact of coinfections on VP-FS, which was not examined in the comparative research studies.
A limitation of case ascertainment from sentinel tertiary pediatric hospitals is that it may not be representative of all FSs in Australia. Differences in health care–seeking behavior could also contribute to bias. Patients with existing medical conditions may be more likely to present for assessment, and families who are familiar with FS may not. However, as we examined first FS only, we feel that this bias is less likely than for subsequent FS.
Given the small proportion of VP-FS cases and limited cohort size, the study would have been able to detect a true difference in the proportion of prolonged seizure in the VP-FS group if it was double the 11.9% in the NVP-FS group, with a power of 0.8. The 6.0% difference between the groups, however, would not be considered clinically significant. Finally, our follow-up data are limited by the high proportion lost to follow-up and short duration. Although it is unclear if there are any differences between those who responded and those who did not, the response rates between NVP-FS and VP-FS were comparable. Larger studies with a longer follow-up period would be useful in confirming our findings and improving recurrence rate estimates.
Conclusions
This study confirms that VP-FSs are clinically not any different from NVP-FSs and should be managed the same way. Our findings can be used to counsel concerned parents that although some vaccines have a known associated risk of FSs, clinical severity and outcomes of these FSs are no different to an FS from another cause. This information helps support the recommendation to these patients and their families that additional required vaccinations can be administered in the future.