Vaccines often contain preservatives, adjuvants, additives, or manufacturing residuals in addition to pathogen-specific immunogens. Some parents, alerted by stories in the news media or information contained on the World Wide Web, are concerned that some of the substances contained in vaccines might harm their children.
We reviewed data on thimerosal, aluminum, gelatin, human serum albumin, formaldehyde, antibiotics, egg proteins, and yeast proteins. Both gelatin and egg proteins are contained in vaccines in quantities sufficient to induce rare instances of severe, immediate-type hypersensitivity reactions. However, quantities of mercury, aluminum, formaldehyde, human serum albumin, antibiotics, and yeast proteins in vaccines have not been found to be harmful in humans or experimental animals.
Vaccines contain live viruses, killed viruses, purified viral proteins, inactivated bacterial toxins, or bacterial polysaccharides. In addition to these immunogens, vaccines often contain other substances. For example, vaccines may contain preservatives that prevent bacterial or fungal contamination (eg, thimerosal); adjuvants that enhance antigen-specific immune responses (eg, aluminum salts); or additives that stabilize live, attenuated viruses (eg, gelatin, human serum albumin). Furthermore, vaccines may contain residual quantities of substances used during the manufacturing process (eg, formaldehyde, antibiotics, egg proteins, yeast proteins).
Some parents, alerted by stories in the news media or on the World Wide Web, are concerned that substances such as thimerosal, formaldehyde, aluminum, antibiotics, and gelatin are harmful. We review safety data obtained from human exposure and experimental animal studies that address these concerns.
PRESERVATIVES
Preservatives are used in some vaccines to prevent bacterial or fungal contamination. The requirement for preservatives in vaccines arose from many incidents in the early 20th century of children who developed severe and occasionally fatal bacterial infections after administration of vaccines contained in multidose vials. For example, in 1916, 4 children died, 26 developed local abscesses, and 68 developed severe systemic infections after receipt of a typhoid vaccine contaminated with Staphylococcus aureus. As a consequence of this and similar incidents, preservatives have been required for vaccines contained in multidose vials (with some exceptions) since the 1930s.
Three preservatives are used in vaccines licensed in the United States: phenol, 2-phenoxyethanol, and thimerosal (Table 1).
Thimerosal, a mercury-containing preservative, has been the focus of intense scrutiny by the US Congress and the news media after its removal from most childhood vaccines in 2001. Attention by the news media has caused some parents to fear that thimerosal contained in vaccines might harm their children.
Removal of thimerosal from vaccines was precipitated by an amendment to the Food and Drug Administration (FDA) Modernization Act, which was signed into law on November 21, 1997.

The amendment gave the FDA 2 years to “compile a list of drugs and foods that contain intentionally introduced mercury compounds and … [to] provide a quantitative and qualitative analysis of the mercury compounds in the list… . ” The amendment arose from a long-standing interest in lessening human exposure to mercury, a known neurotoxin and nephrotoxin.
At the time the FDA Modernization Act was passed, it was recommended that infants receive 3 different vaccines that contained thimerosal: diphtheria-tetanus-acellular pertussis (DTaP), hepatitis B, and Haemophilus influenzae type B (Hib). Infants who received all of these vaccines could have been exposed to a cumulative dose of mercury as high as 187.5 μg by 6 months of age. This value exceeded guidelines recommended by the Environmental Protection Agency (EPA) but did not exceed those recommended by the Agency for Toxic Substances Disease Registry (ATSDR) or the FDA (Table 2). Therefore, thimerosal was removed from most childhood vaccines by 2001 as a precautionary measure.
Although no published studies to date have compared the incidence of neurodevelopmental delay in children who received thimerosal-free or thimerosal-containing vaccines, several facts are reassuring that the level of mercury contained in vaccines was not likely to be harmful. Thimerosal contains 49.6% mercury by weight and is metabolized to ethylmercury and thiosalicylate. Ethylmercury is contained in many drugs as well as biologicals. Adults and children who are exposed inadvertently to large quantities of ethylmercury acutely (quantities 1000- to 1 000 000-fold greater than those found in vaccines) can sustain permanent neurologic damage and death. However, no data exist on the capacity of low-dose, chronic exposure to ethylmercury to harm the developing nervous system. Guidelines for chronic exposure to ethylmercury were extrapolated from guidelines for methylmercury (the most common form of mercury found in the environment) established by the EPA, ATSDR, and FDA.
Guidelines from the EPA were based in part on data from pregnant women in rural Iraq who were exposed to large quantities of methylmercury. In October 1971, Iraq imported >90 000 metric tons of methylmercury-treated seed grain. The grain, distributed free of charge to farmers throughout the country, was used to make bread. Consumption of this bread caused an extensive outbreak of methylmercury poisoning, resulting in >6000 hospitalizations and 450 deaths. By examining the quantity of methylmercury contained in hair from mothers who ingested methylmercury and comparing calculated exposures to methylmercury with the frequency of neurologic symptoms in their offspring (eg, psychomotor retardation, seizures, impaired vision or hearing), a dose-response curve for fetal exposure to methylmercury and neurologic damage was established. The EPA determined guidelines by taking the lowest quantity of methylmercury that might have resulted in harm to the fetus, bracketing that dose with 95% confidence intervals and dividing the lower confidence interval by an “uncertainty” factor of 10.
By using data from pregnant women in Iraq who were exposed to methylmercury in the environment to establish guidelines for chronic exposure of infants in the United States to ethylmercury in vaccines, 2 important assumptions were made: 1) that the toxicity and pharmacokinetics of methylmercury are the same as those of ethylmercury and 2) that the central nervous systems of the fetus and newborn are equally susceptible to the harmful effects of mercury. However, the pharmacokinetics of ethylmercury and methylmercury are not the same. Methylmercury has a biological half-life in blood of approximately 50 days compared with that of approximately 7 days for ethylmercury. Because ethylmercury is excreted from the body far more quickly than methylmercury, cumulative dose guidelines would be very different. In support of this important difference, Pichichero et al found that the level of mercury detected in the blood of 40 full-term infants who were 6 months of age or younger and received thimerosal-containing DTaP, hepatitis B, and Hib vaccines did not exceed recommended guidelines. Furthermore, the developing central nervous system of the fetus is more susceptible to environmental and toxic insults than that of the newborn.
Removal of thimerosal from most vaccines caused several unanticipated consequences. First, before the availability of thimerosal-free DTaP, hepatitis B, and Hib vaccines, hospitals were advised to defer the birth dose of hepatitis B vaccine to 2 to 6 months of age in infants of hepatitis B-seronegative mothers. Some hospitals misinterpreted this guideline and suspended administration of the birth dose of hepatitis B vaccine for all newborns. As a consequence, 1 institution reported that 3 infants of hepatitis B-seropositive mothers did not receive the recommended birth dose of hepatitis B vaccine. Another institution reported the death from acute hepatitis B-induced liver failure of a 3-month-old infant who was born to a hepatitis B-seropositive mother; the infant did not receive the hepatitis B vaccine. Furthermore, although thimerosal-free vaccines are now available, many hospitals continue to defer the birth dose of hepatitis B vaccine inappropriately. Second, the removal of thimerosal from vaccines caused some parents and physicians to believe that vaccines that contain thimerosal were harmful, independent of dose or age of administration. For example, although contrary to recommendations by the Centers for Disease Control and Prevention, some parents and physicians were hesitant to give any thimerosal-containing vaccines to children (eg, influenza vaccine to children at high risk of severe influenza infection). Third, although thimerosal was removed from vaccines in part to “maintain the public’s trust in immunization,” some physicians found that parents were less confident in professional groups that recommended vaccines before than after removal of thimerosal.
ADJUVANTS
Aluminum salts are the only adjuvants currently licensed for use in the United States (Table 3). Aluminum salts include aluminum hydroxide, aluminum phosphate, and potassium aluminum sulfate (alum). Aluminum-containing vaccines are prepared by adsorption of antigens onto aluminum hydroxide or aluminum phosphate gels or by precipitation of antigens in a solution of alum.
Aluminum salts were found initially to enhance immune responses after immunization with diphtheria and tetanus toxoids in studies performed in the 1930s, 1940s, and 1950s. Early studies suggested that aluminum salts reduced the rate of elimination of antigens at the site of inoculation (ie, depot effect). However, subsequent studies questioned the importance of the depot effect and found that aluminum salts enhanced antigen uptake by antigen-presenting cells (eg, dendritic cells), activated antigen-presenting cells, or induced production of cytokines and complement. The importance of each of these mechanisms in enhancing antigen-specific immune responses remains unclear.
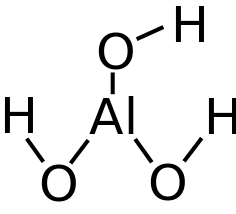
The safety of aluminum has been established by experience during the past 70 years, with hundreds of millions of people inoculated with aluminum-containing vaccines. Adverse reactions including erythema, subcutaneous nodules, contact hypersensitivity, and granulomatous inflammation have been observed rarely.
Aluminum-containing vaccines are not the only source of aluminum exposure for infants. Because aluminum is 1 of the most abundant elements in the earth’s crust and is present in air, food, and water, all infants are exposed to aluminum in the environment. For example, breast milk contains approximately 40 μg of aluminum per liter, and infant formulas contain an average of approximately 225 μg of aluminum per liter. Vaccines contain quantities of aluminum similar to those contained in infant formulas (Table 3). However, because large quantities of aluminum can cause serious neurologic effects in humans, guidelines were established by the ATSDR.
For determining the quantity of aluminum below which safety is likely, data were generated in mice that were inoculated orally with various quantities of aluminum lactate. No adverse reactions were observed when mice were fed quantities of aluminum as high as 62 mg/kg/day. By applying uncertainty factors of 3 (for extrapolation to humans) and 10 (for human variability), the ATSDR concluded that the minimum risk level for exposure to aluminum was 2 mg/kg/day. The half-life of elimination of aluminum from the body is approximately 24 hours. Therefore, the burden of aluminum to which infants are exposed in food and vaccines (Table 3) is clearly less than the guideline established by the ATSDR and far less than that found to be safe in experimental animals.
ADDITIVES
Additives are used to stabilize vaccines from adverse conditions such as freeze-drying or heat. In addition, additives are added to vaccines to prevent immunogens from adhering to the side of the vial. The types of stabilizers used in vaccines include sugars (eg, sucrose, lactose), amino acids (eg, glycine, monosodium salt of glutamic acid), and proteins (eg, gelatin or human serum albumin).
Three issues surround the use of protein additives in vaccines: 1) the observation that immediate-type hypersensitivity reactions are a rare consequence of receiving gelatin-containing vaccines, 2) the theoretical concern that human serum albumin might contain infectious agents, and 3) the theoretical concern that bovine-derived materials used in vaccines might contain the agent associated with bovine spongiform encephalopathy (“mad-cow” disease).
Hypersensitivity to Gelatin
In 1993, Kelso et al reported the case of a 17-year-old girl in California who developed profuse rhinorrhea, hives, laryngotracheal edema, lightheadedness, and a blood pressure of 70/50 within 5 minutes of receiving a measles-mumps-rubella (MMR) vaccine. Her symptoms resolved after treatment with epinephrine and diphenhydramine. When later describing the event, the girl stated that it was “kind of like what happens when I eat Jell-O.” Subsequent testing found that the only component of the vaccine to which the patient was allergic was gelatin.
Before 1993, immediate-type hypersensitivity reactions to the MMR vaccine were attributed to an allergy to egg proteins. This assumption was based on the fact that both the measles and mumps components of MMR vaccine are grown in chick embryo fibroblast cells. However, most patients with hypersensitivity to MMR vaccine were not allergic to eggs. The observation by Kelso et al prompted a closer look at the capacity of gelatin-containing vaccines to induce hypersensitivity reactions.
Studies in Japan confirmed the findings of Kelso et al that immediate hypersensitivity to MMR vaccine was associated with the presence of gelatin-specific immunoglobulin E (IgE), not an allergy to egg proteins. At that time, the rate of immediate hypersensitivity to MMR in Japan was approximately 20-fold higher than that in the United States. The increased incidence of immediate-type hypersensitivity to gelatin in Japan was explained in 2 ways. First, DTaP vaccines made in Japan contained gelatin, whereas DTaP vaccines made in the United States did not. Second, the type of gelatin used in Japan was not hydrolyzed. Hydrolysis converts high molecular weight gelatin (>100 000 Da) to low molecular weight gelatin (between 2000 and 5000 Da). Low molecular weight gelatin is less likely to stimulate gelatin-specific IgE than high molecular weight gelatin. When Japanese vaccine makers eliminated gelatin from DTaP and switched to the use of hydrolyzed gelatin in the MMR vaccine, the incidence of gelatin-specific immediate-type hypersensitivity reactions decreased dramatically to levels similar to those found in the United States.
Although the incidence of anaphylaxis to gelatin is currently very low (approximately 1 case per 2 million doses), gelatin is the most common identifiable cause of immediate-type hypersensitivity reactions to gelatin-containing vaccines. A list of vaccines that contain gelatin is provided in Table 4 (all gelatin is of porcine origin).
Some patients with immediate hypersensitivity reactions to gelatin have a history of allergies to gelatin-containing foods. This is explained, in part, by the extensive cross-reactivity found between bovine gelatin contained in many foods and porcine gelatin contained in vaccines. Therefore, it would be of value to ask about food allergies before vaccination with gelatin-containing vaccines. If children have either a history of food allergy to gelatin or a history of immediate-type hypersensitivity reactions to gelatin-containing vaccines, then gelatin-containing vaccines should not be administered and an immunologic evaluation should be performed. Evaluation may include either detection of gelatin-specific IgE by solid-phase immunoassay or skin testing with increasing concentrations of gelatin. Vaccination of people who have immunologic evidence for gelatin hypersensitivity should be performed with the ready availability of equipment and medications required for the treatment of anaphylactic reactions or deferred completely.
Theoretical Risk of Infectious Agents in Human Serum Albumin
Human serum albumin (0.3 mg/dose) is contained in measles vaccine (Attenuvax; Merck and Co, West Point, PA); mumps vaccine (Mumpsvax; Merck and Co); rubella vaccine (Meruvax; Merck and Co); and measles, mumps, and rubella vaccine (MMRII; Merck and Co).
Because human serum albumin is derived from human blood, there is a theoretical risk that it might contain infectious agents. However, the FDA requires that human serum albumin be derived from blood of screened donors and be manufactured in a manner that would eliminate the risk of transmission of all known viruses. The result is that no viral diseases have ever been associated with the use of human serum albumin.
Theoretical Risk of “Mad-Cow” Disease From Bovine-Derived Reagents
Creutzfeld-Jacob disease (CJD) in humans is caused by a unique infectious agent (proteinaceous infectious particles, or prions) that also causes encephalopathies in other mammals such as cows (bovine spongiform encephalopathy [BSE]) and sheep (scrapie).
Between 1995 and 1997, a new “variant” form of CJD (vCJD) in humans was reported from the United Kingdom after an outbreak of BSE in cows. The timing of these events raised the possibility that people who ate products from cows that were infected with BSE developed vCJD. Several epidemiologic, clinical, and pathologic features of vCJD supported a causal link between BSE and vCJD.

Vaccines contain several reagents that are derived from cows (eg, gelatin, glycerol, enzymes, serum, amino acids). Because of concerns about vCJD, the FDA recently prohibited the use of bovine-derived materials obtained from countries that are known to have cattle that are infected with BSE. However, before this ban, some materials used in vaccines might have been obtained from cows that were infected with BSE in England. (It should be noted that US vaccine manufacturers were not allowed to use bovine products imported from outside the United States to protect against the possible importation of foot-and-mouth disease).
This raised the question of whether children who were inoculated with vaccines were at risk for vCJD. Newspapers reported this possibility in the late 1990s, and some parents were concerned about bovine-derived products contained in vaccines. However, several epidemiologic observations and features of the manufacturing process should reassure parents that vaccines could not cause vCJD.
First, prions are detected in the brain, spinal cord, and retina of cows with BSE and not in blood or other organs. Therefore, serum (present in media that support the growth of microorganisms or cells used to make vaccines) is not likely to contain prions. Consistent with these observations, no cases of CJD have been transmitted by blood or blood products, and a history of blood transfusion does not increase the risk for CJD.
Second, prions are not detected in connective tissue of cows with BSE. Therefore, gelatin (made by boiling the hooves and skin of pigs or cows) is unlikely to contain prions.
Third, epidemiologic evidence does not support vaccines as a cause of vCJD in England. Human exposure to cows that were infected with BSE in England was likely to have occurred after 1983, and vaccines that contained bovine-derived materials were likely to have been administered to children after 1985. If vaccines that are routinely administered in the first 2 years of life caused vCJD, then no cases of vCJD would have been expected to occur in people who were born before 1985. However, all cases of vCJD occurred in people born who were before 1985 and half before 1970.
MANUFACTURING RESIDUALS
Residual quantities of reagents that are used to make vaccines are clearly defined and well regulated by the FDA. Inactivating agents (eg, formaldehyde), antibiotics, and cellular residuals (eg, egg and yeast proteins) may be contained in the final product.
Inactivating Agents
Inactivating agents separate a pathogen’s immunogenicity from its virulence by eliminating the harmful effects of bacterial toxins or ablating the capacity of infectious viruses to replicate. Examples of inactivating agents include formaldehyde, which is used to inactivate influenza virus, poliovirus, and diphtheria and tetanus toxins; β-propiolactone, which is used to inactivate rabies virus; and glutaraldehyde, which is used to inactivate toxins contained in acellular pertussis vaccines. Formaldehyde deserves special consideration.
Concerns about the safety of formaldehyde have centered on the observation that high concentrations of formaldehyde can damage DNA and cause cancerous changes in cells in vitro. Although formaldehyde is diluted during the manufacturing process, residual quantities of formaldehyde may be found in several current vaccines (Table 5). Fortunately, formaldehyde does not seem to be a cause of cancer in humans, and animals that are exposed to large quantities of formaldehyde (a single dose of 25 mg/kg or chronic exposure at doses of 80–100 mg/kg/day) do not develop malignancies.
The quantity of formaldehyde contained in individual vaccines does not exceed 0.1 mg (Table 5). This quantity of formaldehyde is considered to be safe for 2 reasons. First, formaldehyde is an essential intermediate in human metabolism and is required for the synthesis of thymidine, purines, and amino acids. Therefore, all humans have detectable quantities of formaldehyde in their circulation (approximately 2.5 μg of formaldehyde/mL of blood). Assuming an average weight of a 2-month-old of 5 kg and an average blood volume of 85 mL/kg, the total quantity of formaldehyde found naturally in an infant’s circulation would be approximately 1.1 mg—a value at least 10-fold greater than that contained in any individual vaccine. Second, quantities of formaldehyde at least 600-fold greater than that contained in vaccines have been given safely to animals.
Antibiotics
Antibiotics are present in some vaccines to prevent bacterial contamination during the manufacturing process. Because antibiotics can cause immediate-type hypersensitivity reactions in children, some parents are concerned that antibiotics that are contained in vaccines might be harmful. However, antibiotics that are most likely to cause immediate-type hypersensitivity reactions (eg, penicillins, cephalosporins, sulfonamides) are not contained in vaccines.
Antibiotics that are used during vaccine manufacture include neomycin, streptomycin, polymyxin B, chlortetracyline, and amphotericin B. Only neomycin is contained in vaccines in detectable quantities (Table 6). However, immediate-type hypersensitivity reactions to the small quantities of neomycin contained in vaccines has not been clearly documented. Although neomycin-containing products have been found to cause delayed-type hypersensitivity reactions, these reactions are not a contraindication to receiving vaccines.
Cellular Residuals
Egg Proteins
Egg allergies occur in approximately 0.5% of the population and in approximately 5% of atopic children. Because influenza and yellow fever vaccines both are propagated in the allantoic sacs of chick embryos (eggs), egg proteins (primarily ovalbumin) are present in the final product. Residual quantities of egg proteins found in the influenza vaccine (approximately 0.02–1.0 μg/dose) are sufficient to induce severe and rarely fatal hypersensitivity reactions in children with egg allergies. Unfortunately, children with egg allergies also have other diseases (eg, asthma) that are associated with a high risk of severe and occasionally fatal influenza infection. For this reason, children who have egg allergies and are at high risk of severe influenza infection should be given influenza vaccine via a strict protocol.
In contrast to influenza vaccine, measles and mumps vaccines are propagated in chick embryo fibroblast cells in culture. The quantity of residual egg proteins found in measles- and mumps-containing vaccines is approximately 40 pg—a quantity at least 500-fold less than those found for influenza vaccines. The quantity of egg proteins found in measles- and mumps-containing vaccines is not sufficient to induce immediate-type hypersensitivity reactions, and children with severe egg allergies can receive these vaccines safely.
Yeast Proteins
In contrast to influenza vaccine, measles and mumps vaccines are propagated in chick embryo fibroblast cells in culture. The quantityHepatitis B vaccines are made by transfecting cells of Saccharomyces cerevisiae (baker’s yeast) with the gene that encodes hepatitis B surface antigen, and residual quantities of yeast proteins are contained in the final product. Engerix-B (GlaxoSmithKline, Research Triangle Park, NC) contains no more than 5 mg/mL and Recombivax HB (Merck and Co) contains no more than 1 mg/mL yeast proteins.
Immediate-type hypersensitivity reactions have been observed rarely after receipt of hepatitis B vaccine (approximately 1 case per 600 000 doses). However, yeast-specific IgE has not been detected in patients with immediate-type hypersensitivity or in nonallergic patients after receipt of hepatitis B vaccine. Therefore, the risk of anaphylaxis after receipt of hepatitis B vaccine as a result of allergy to baker’s yeast is theoretical. of residual egg proteins found in measles- and mumps-containing vaccines is approximately 40 pg—a quantity at least 500-fold less than those found for influenza vaccines. The quantity of egg proteins found in measles- and mumps-containing vaccines is not sufficient to induce immediate-type hypersensitivity reactions, and children with severe egg allergies can receive these vaccines safely.
CONCLUSION
Parents should be reassured that quantities of mercury, aluminum, and formaldehyde contained in vaccines are likely to be harmless on the basis of exposure studies in humans or experimental studies in animals. Although severe anaphylactic reactions may occur rarely after receipt of vaccines that contain sufficient quantities of egg proteins (eg, influenza, yellow fever) or gelatin (eg, MMRII), children who are at risk for severe infection with influenza can be desensitized to influenza vaccine, and gelatin-specific allergies are very rare. Immediate-type hypersensitivity reactions to neomycin or yeast proteins have not been clearly documented and remain theoretical.
Acknowledgments
Some vaccines discussed in this article are manufactured by Merck and Co. Dr Offit is the co-holder of a patent on a bovine-human reassortant rotavirus vaccine that is being developed by Merck. Dr Offit’s laboratory support comes from the National Institutes of Health, and he does not receive personal support or honoraria from Merck and does not have a financial interest in the company.